

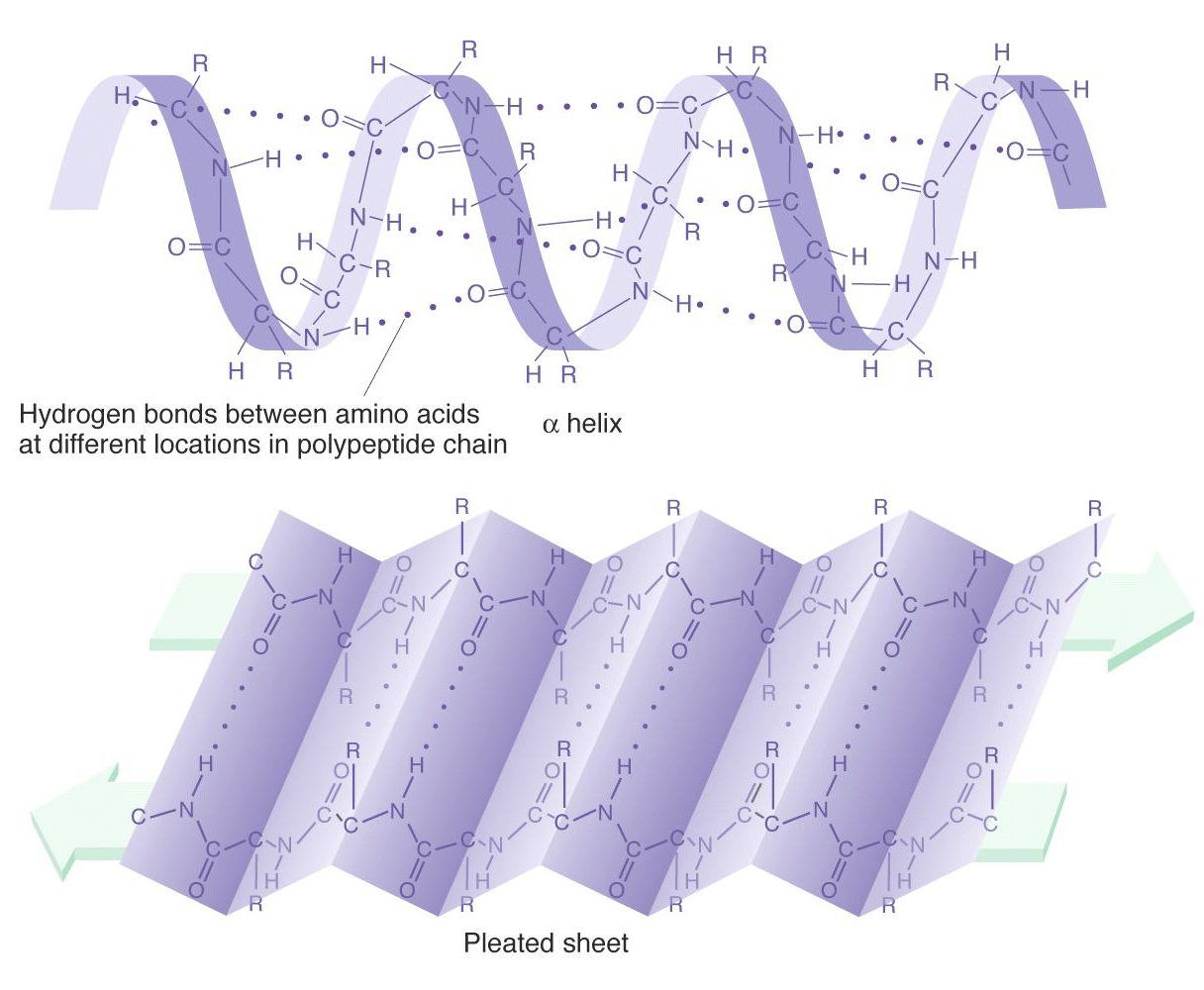
Īnother practical application is the development of thermostable proteins that incorporate D-amino acids. Methods are being developed for incorporating L and D amino acids in computational de novo protein design. Other microbial peptides such as tolaasin use D-amino acids to enforce sharp bends in an α-helical domain. The β-helix has been used as the foundation for novel cyclic peptide folds and peptide nanotubes with ion channel activity and antimicrobial properties. This allows it to adopt the β-helix, a novel secondary structure composed of alternating positions in the β L and β R conformation. The antimicrobial toxin, gramicidin, is a well studied example of such a molecule, containing alternating L and D amino acids. This work has been motivated by natural examples of polypeptides that combine L and D amino acids. Much of the work on the role of variable stereochemistry on structure and stability has been conducted on short peptides. In this study, we use the Protein Data Base (PDB) as a source of structural information for specific D-amino acid sidechain interactions with α-helical backbones. The resulting enantiomeric molecules were both well-folded and specifically active on a protease substrate of the same respective amino acid chirality as the enzyme. A dramatic example was the chemical synthesis of the ninety-nine amino acid long HIV-1 protease from both L and D-amino acids. This has allowed protein chemists to explore the physical and biological effects of varying amino acid stereochemistry. The field has developed rapidly, permitting the construction of synthetic, protein-sized molecules. Solid phase chemical synthesis allows for the incorporation of non-natural amino acids into polypeptides. These will provide a basis for de novo design of novel heterochiral protein folds. Conclusionīy examining left-handed α-turns containing L-amino acids, new interaction motifs for incorporating D-amino acids into right-handed α-helices are identified. Helix capping sidechain-backbone interactions are found which are unique to α L helices including an elevated propensity for L-Asn, and L-Thr at the amino terminus and L-Gln, L-Thr and L-Ser at the carboxy terminus. Two backbone rules for terminating a left-handed helix are found: an α R conformation is disfavored at the amino terminus, and a β R conformation is disfavored at the carboxy terminus. Propensities for amino acids to occur in contiguous α L helices correlate with published thermodynamic scales for incorporation of D-amino acids into α R helices. Molecular mechanisms used in proteins to stabilize left-handed elements by L-amino acids are structurally enantiomeric to potential synthetic strategies for stabilizing right-handed elements with D-amino acids. However, protein elements containing short left-handed turns and helices turn out to contain useful information. D-amino acids rarely occur naturally, making it difficult to infer general rules for how they would be tolerated in proteins through an analysis of existing protein structures. The Protein Data Bank has been a reliable, rich source of information on molecular interactions and their role in protein stability and structure.

Incorporating variable amino acid stereochemistry in molecular design has the potential to improve existing protein stability and create new topologies inaccessible to homochiral molecules.


 0 kommentar(er)
0 kommentar(er)
